Steraloids Steroid Naming Convention
This information should help you locate a compound in our catalogue. Please do not hesitate to call us if this is confusing.
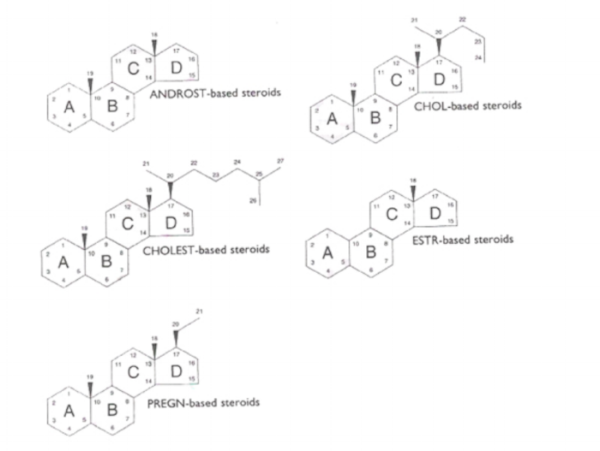
Step 1
Begin by choosing a root name for the structure that most closely matches the molecule shown. The root name will be prefixed and suffixed to show the differences between the actual compound and the idealized structure.
These 5 examples are the 5 most common structures in our catalogue.
Step 2
Now add a root suffix denoting the number of unsaturated (double) bonds from the table. For example: to show that there are no double bonds ass the suffix 'AN'. If there are no items following the root name, or if the root is followed by a consonant you should add a further 'E'. It helps to remember the suffixes if you think of each double bond adding an 'EN'.
Step 3
Having said how many unsaturated bonds there are, now you need to specify where they are.
Bonds are numbered in terms of the lowest number of the two positions, so for example a double bond between two carbons at positions 2 and 3 would simply be labeled '2'. There is no ambiguity in this case since a bond between 2 and 1 would be labeled '1'.
However, sometimes there is the possibility of ambiguity, for example involving double bonds along the ring junctions (positions 5,8,9,10, and 14). In these cases, the second position should be included in brackets, so a bond between positions 5 and 10 would be labelled as '5(10)' and one between 5 and 6 as '5'.
Ambiguities are most likely to occur amongst Estrogens where the '10' position is missing the usual methyl group.
5𝛂-ANDROSTANE
This compound has no saturated bonds.
4-ANDROSTEN-3𝛂, 17𝛃-DIOL
This compound has a single unsaturated bond between positions 4 and 5
3,5,-PREGNADIEN-20-ONE
This compound has two unsaturated bonds between positions 3 & 4 and 5 & 6. There is no ambiguity with the 5(6) bond, since position 10 is occupied by the methyl group and is therefore unavailable to form a double bond with position 5.
1,4,9-ANDROSTATRIEN-3, 17-DIONE
This compound has three unsaturated bonds, between positions 1 & 2, positions 4 & 5, and positions 9 & 11. There is no ambiguity with the 9(11) bond, since the methyl group once again occupies position 10.
1,3,5(10), 8(9) -ESTRATETRAEN-3-OL-ONE
This compound has four unsaturated bonds between positions 1 & 2, 3 & 4, 5 & 10, and 8 & 9. The 8(9) bond is specified to remove any ambiguity with possible double bond at 8(14).
Step 4
Prefix your compound name with the positions and names for any structural elements.
Structural elements include the orientation of methyl groups (𝛂 or 𝛃), expanded (HOMO) or contracted (NOR) rings, multiple structurqal omissions, e.g. missing side-chains from cholan- and cholest- compounds (BISNOR), and broken rings (SECO).
If necessary, to avoid confusion with unsaturated bond numberings, bracket the positions of orientative 𝛂's or 𝛃's as in these examples:
5𝛂-PREGNANE
5𝛂 indicates that the hydrogen at 5 is in the 𝛂 orientation - effectively "into the page" as opposed to 𝛃 "out of the page". See compound PI800-000
1, (5𝛂)-ANDROTEN-3, 17 DIONE
Here, the 5𝛂 is bracketed to prevent the suggestion of a double bond at 5(6). See compound A4400-000
D-HOMO-5𝛂-ANDROSTQAN-17𝛂-METHYL-3𝛃,17𝛂𝛃-DIOL
D-HOMO indicates that the 'D' ring has been expanded to a six membered ring, renumbered as 13,14,15,16,17,17𝛂, and 18. See compound H4080-000.
19-NOR-4PREGNEN-3,20-DIONE
19-NOR indicates that the C19 is missing. See compound N1500-000.
A-NOR5𝛂-ANDROSTAN-2𝛂,17𝛂-DIETHYNYL-2𝛃,17𝛃-DIOL DIPROPIONATE
A-NOR indicates that the 'A' ring is a five membered ring, with the carbons renumbered as 1,2,3, and 5. See compound N0100-000.
23,24-BISNOR-5-CHOLENIC ACID-3𝛃-OL
23,24-BISNOR indicates that both the 23 and 34 carbon atoms of the cholenic sidechain have been removed., See compound B0700-000.
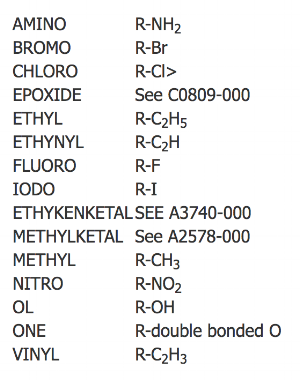
Step 5
Then add suffixes that show the positions, orientations, and types of the functional groups (left) or substituted positions in numerical/alphabetical order.
Multiple substitutions and derivatives are prefixed with a multiplier; DI (x2), TRI (x3), TETR or TETRA (x4), PENT or PENTA (x5).
e.g. DIBROMO, DIONE, TRI-ETHYL, TETRA-FLUORO, PENTA-CHLORO, etc,.
Step 6
Finally, if the compound is a standard derivative of another compound, add the derivative name. On the left is a list of common derivatives, giving both the additions to the original compound's formula, and the change in its molecular weight.